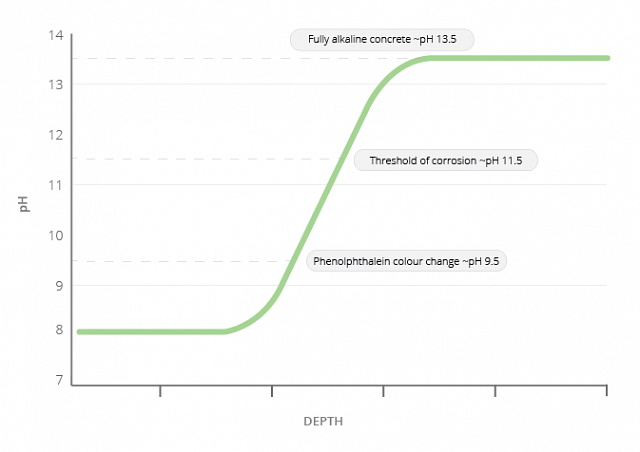
Carbonation is a simpler process than chloride attack. Atmospheric carbon dioxide reacts with the pore water to form carbonic acid. This reacts with the calcium (and other) hydroxides to form solid carbonates. The pH therefore drops from around pH 12 to around pH 8. The steel starts to corrode at around pH 11.
Carbonation is associated with poor concrete cover, poor concrete quality, poor consolidation and old age in the absence of chlorides. Carbonated concrete is good quality concrete but it is no longer protective to the reinforcing steel. Carbonation rates generally follow parabolic kinetics:
d = At0.5
where :
d = carbonation depth
t = time
A is a constant, generally of the order 0.25 to 1.0mm.year-½
Carbonation rates are a function of the environment where indoor concrete will carbonate faster than outdoor concrete in North American and Northern European environments, but wet/dry cycling in warm conditions can accelerate carbonation in more southerly latitudes. As corrosion will not proceed in the absence of water, carbonation rates are usually unimportant inside buildings except in bathrooms, kitchens and other situations where there is wet/dry cycling and sufficient moisture to cause corrosion after carbonation to rebar depth.